

Optimize Your
mRNA Manufacturing
The SaniSure Advantage
We understand that your mRNA and LNP manufacturing processes are unique in their scale, specific needs and final indication requirements.
As your ideal partner and go-to experts for small scale GMP processes, SaniSure has specific and optimized process solutions for small scale pDNA, mRNA and LNP production.
We support your specific GMP manufacturing process with optimized solutions for:
- Closed processing and process liquid transfer
- Intermediate mixing, storage and shipping
- Vials for stability studies, release and retain samples
- Final filling needles and assemblies
Your Ideal End-to-End Partner for mRNA Manufacturing
Plasmid DNA Production and Purifications
SaniSure’s unique products, including Mixed4Sure™, PharmaTainer™ and One2Fill™ support the mixing, high-value reagent preparation, and intermediate storage needs of your plasmid DNA Process.
Provide your chromatography buffers in a contained set-up
Mixed4Sure™
A unique solution for closed, small volume mixing in GMP processing.
- Ensures thorough mixing of your intermediates, supports enzymatic reactions and preparation of reagents
- Provides continuous mixing of process volumes from 50ml to 20L—significantly lower mixing volumes than bag mixing systems
- Exerts 2X lower shear forces than comparable bag mixing systems
- Is easy to use and integrate into your process
- Configurable to your specific mixing requirements

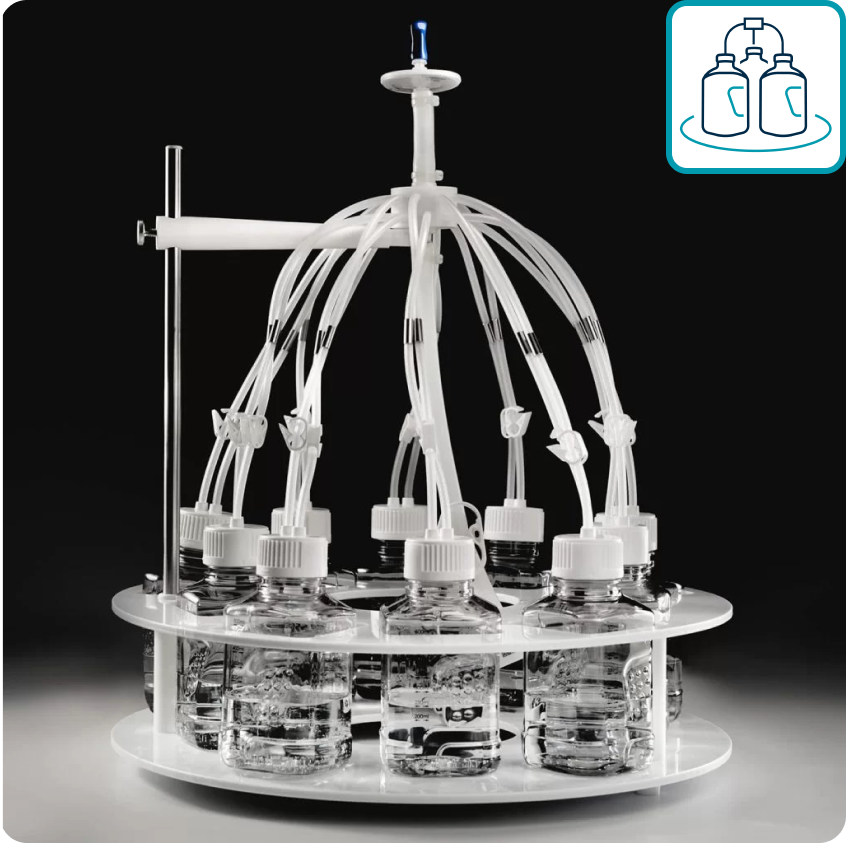
One2Fill™ 10X
A compact, closed and easy-to-implement solution for sterile, multiple bottle filling for closed, small volume mixing in GMP processing.
- Streamlines intermediate, bulk and reagent filling operations during pDNA, mRNA and LNP manufacturing
- Enables 10x faster filling than individual filling and 40-second setup
- Delivers +/- 3% filling accuracy
- Is easy to use and adaptable to your process needs
- Allows for less integrity testing with single vent filter
- Offers industry leading low particle contamination risk and robust bulk filling solution.
- Compact, low CapEx
Fill4Sure™
Bespoke, single-use final fill assemblies designed around your drug product.
- Dedicated assembly design experts follow the process from initial concept to operational support
- Enables multi-line filling without the introduction of downstream line splitting
- Is a reliable second-source option or a new, customized assembly with built-in efficiency and security
- Operates with independent fill lines, providing superior fluid dynamics to ensure the process pumps can perform optimally for accurate drug product filling
- Minimizes operational disruptions with a vertically-integrated supply chain
- Utilizes customized surge bags that can have 1-12 filling ports, depending on your fill requirements
- Uses standard OEM replacement needles and fully customizable needles bespoke to drug product considerations, and for any type of filling machine, produced without adhesives or oils for minimal particle generation


PharmaTainer™
- The world’s cleanest bottle
- PET or PC materials
- Individual bottle traceability with serial number, lot number and expiration date
- Extremely low particulates < 1.7% of USP <788> LVP limits
- Bottle molding and capping under laminar airflow/ISO 5 One-piece, gasket-free, molded double-seal cap
- Extensively characterized
Stability Vials
- Ideal for release, retain and stability study samples
- Uses same resins as larger volumes for representative results
- HDPE cap with a double seal system
- Certified sterile SAL 10-6
- Cap torque chuck available
Bottle Assemblies
- Designed for complex flow paths
- Open architecture
- Available in polypropylene, PVDF, polyethylene, polysulfone, fluoropolymers, polycarbonate and other materials based on individual application needs

Closed System Process Vessels
- Designed to close complex flow paths
- Used primarily in critical applications
- Low particles, high robustness
- One-piece molded and barbed caps
Let’s get started.
See how our industry leading solutions can optimize your mRNA manufacturing flow paths.