Home / Our products / Single-Use Components / Bag Systems / Flex4Sure™ 3D Bags
BAG SYSTEMS
Flex4Sure™
3D Bag Solutions
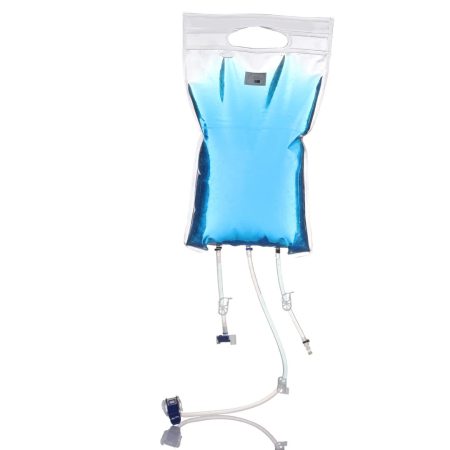
Flex4Sure™ 3D Bag Systems incorporate chambers manufactured from INFUFLEX film from Renolit. This co-extruded film has a PE contact layer and offers robustness, a well-characterized extractable profile, high clarity, and excellent chemical resistance. In sizes from 100L to 1000L, Flex4Sure™ 3D Bag Systems are adapted to fit various existing rigid storage totes. The SaniSure® Flex4Sure™ 3D Bags eliminate CapEx for this hardware as they can be integrated quickly into any current process for storage of fluids used in biopharmaceutical manufacturing. Standard Flex4Sure™ 3D Bags are available with Cellgyn® TPE tubing and MPC connectors for rapid delivery or can be integrated into custom assemblies designed to meet your application.

Overview
Flex4Sure™ 3D Bags
Applications
- Storage and transport of buffers and culture media
- Transport of harvested cell culture fluids
- Purified component collection
- Filtration and storage of intermediates
- Storage and transport of drug substance
- Customized tubing types, sizes, and lengths
- Manifolds can also be specified for additional process security in critical applications.
Benefits
- Highly transparent film materials for enhanced visual inspection of process fluids
- Flexible customization of sizes, tubings, joints, and filters for a variety of biopharmaceutical processes
- Simplify implementation with chambers that are compatible with a variety of support containers/totes
- A wide range of distal connectors (filters, aseptic and hygienic connectors)
Specifications
- 100L – 1000L – 3-port.
- All chambers are available in 2 configurations and compatible with market-leading totes
- Operating Temperature 0 to 60 °C
- Gamma-ray irradiation sterilization (SAL 10-6)
- EX Tear Double-layer PE bag vacuum packaging
- Thickness 0.325 mm offering good flexibility and robustness
- BPOG Extractables package available on request
COMPLIANCE
- ISO 10993-4: Hemolysis
- ISO 10993-5: Cytotoxicity
- ISO 10993-6: Implantation test
- ISO 10993-10: Irritation and Sensitization tests
- ISO 10993-11: Acute Systemic Toxicity test
- USP<85>: Bacterial Endotoxins -LAL test on request
- USP<661>: Plastic Containers European Pharmacopoeia tests
- Ch.3.1.5. USP<88>: Biological reactivity testing, in vivo, class VI.
- USP<661>: Plastic Containers European Pharmacopoeia tests
- Ch.3.1.5. ADCF: No animal derived ingredients for film
More in Bag Systems
Looking to simplify change management?
The Flex4Sure™ 3D Bags offer supply chain flexibility to pharma manufacturers. Learn how we can create custom assemblies designed to meet your applications.