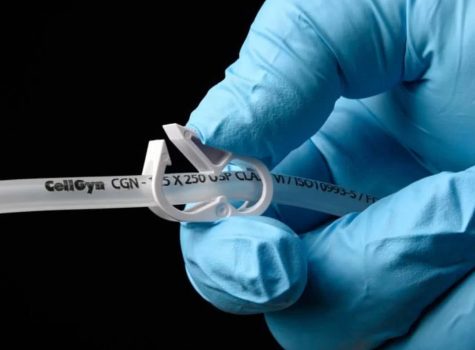
TR-1092 study proves ClearGreen®Pure outperforms leading PVC tubing in weld integrity, burst pressure, and USP <788> compliance for bioprocessing applications.
The News & Events
Strengthen bioprocessing supply chains with SaniSure’s USA-based manufacturing. Eliminate import delays, reduce hidden costs, ensure FDA compliance.
Discover how SaniSure delivered 70 custom assemblies in half the standard lead time, supporting vital viral vector manufacturing for a leading CDMO.
This comprehensive white paper examines the critical performance characteristics of SaniSure’s® CellGyn® tubing in aseptic biopharmaceutical applications.
This white paper explores critical contamination control strategies and advanced containment solutions to protect sensitive mRNA and gene therapies in GMP biopharmaceutical manufacturing environments.
This study validates gamma irradiation sterilization methods for single-use bioprocessing systems, demonstrating effective sterility assurance using VDmax27.5 method.
A comprehensive framework for particulate control and quality assurance in cleanroom manufacturing of bioprocessing systems.
The study addresses the critical challenge of maintaining leak-tight connections in bioprocessing assemblies over extended storage periods.
SaniSure® clamp design overcomes limitations of traditional TC clamps when securing flexible, disposable sanitary fittings in bioprocessing applications.