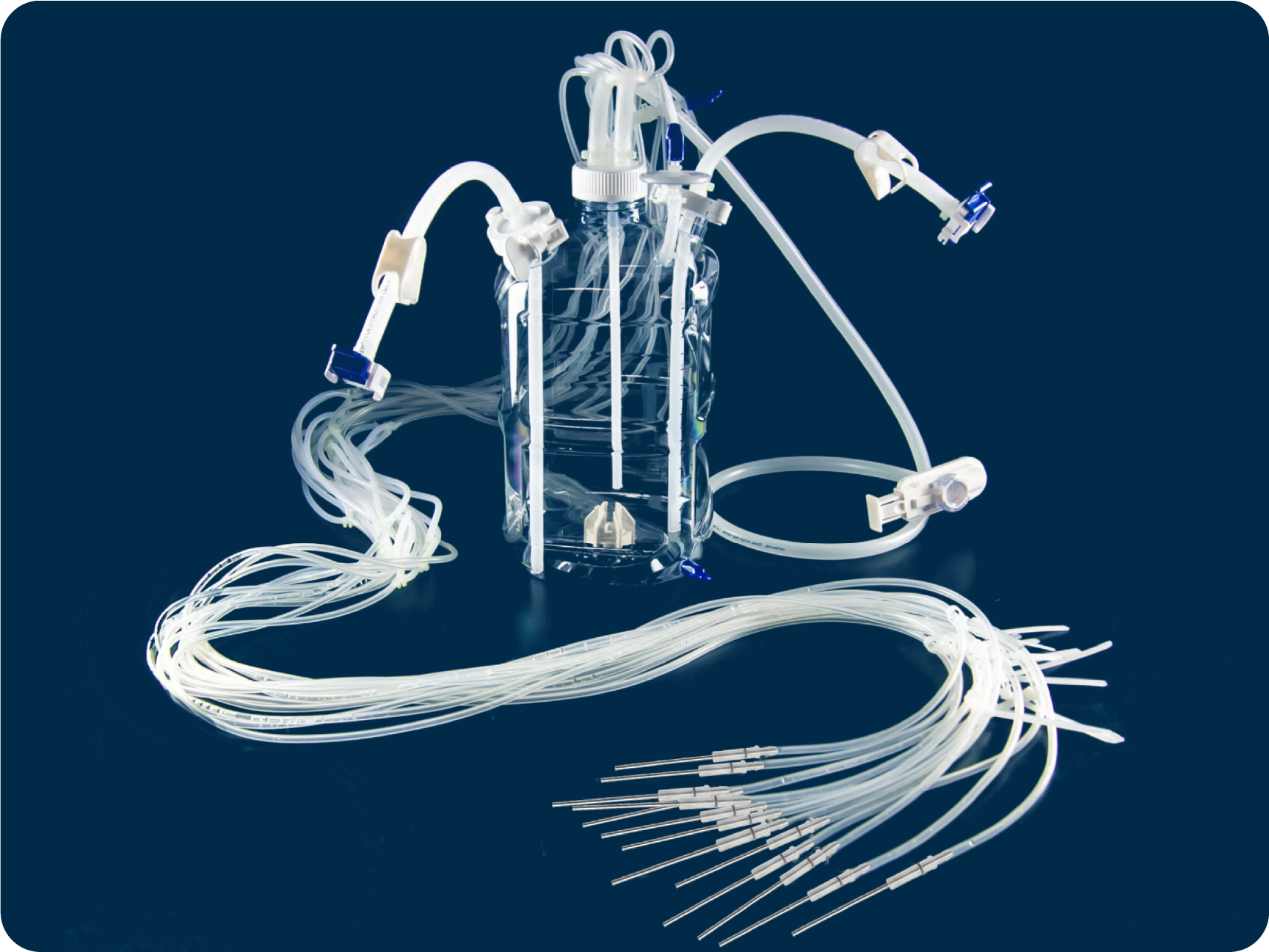
By balancing the User Requirement Specifications you provide with our fluid transfer expertise—such as flow rate, pressure, shear sensitivity, hold-up volume, and sterility requirements—we engineer a closed, optimized flow path using the most appropriate tubing, connectors, manifolds, and filtration components.
Custom FLUID MANAGEMENT
Tailored Single-Use Solutions for Your Complex Applications
At SaniSure, we design, engineer and build custom, best-in-class fluid management assemblies to help optimize your biomanufacturing processes.
SPEED, AGILITY AND EXPERIENCE TO OPTIMIZE YOUR FLOW PATHS
DESIGNED FOR COMPLEX FLOW PATHS
We combine unparalleled expertise in complex flow paths, closed systems design excellence and a vast library of engineered components—all while embracing an open architecture process—to quickly deliver flexible fluid management solutions that work
WE HOLD A VAST LIBRARY OF VALIDATED, ENGINEERED COMPONENTS
From tubing and containers, to flexible mixing and final fill solutions—and everything in between—we offer everything you need to build the single-use fluid management assembly your process demands. This includes proprietary components like PharmaTainerTM—the cleanest bottle on the market, and our Cap2V8® closure systems protecting product integrity and providing an aseptic seal for high-purity biologicals.
OPEN ARCHITECTURE FOR ANY SINGLE-USE FLUID PATH
Beyond our vertically integrated components, we provide an extensive library of qualified third‑party components, giving you a truly open‑architecture approach to designing single‑use fluid transfer paths that meet your specific process and regulatory requirements for chemical compatibility, pressure performance, and other complex process conditions such as sterility, extractables and leachables, and temperature or viscosity limits
DRIVE SUCCESS WITH OUR PROVEN SIX-STEP METHOD
From defining the challenges to delivering ready-to-use solutions, our six-step method is a proven, streamlined process to help you solve your most challenging flow paths. See our six-step process below.
FLUID MANAGEMENT ASSEMBLY CONFIGURATION OPTIONS
Filter Types
Validated library of sterilizing filters in varying pore sizes and surface areas.
Filter AssembliesConnect with a SaniSure representative to build your fluid management solution today.
LET'S GET STARTEDAPPLICATIONS
Your complex flow paths and critical applications deserve optimized solutions.
ALL FLUID MANAGEMENT SOLUTIONS












Mixing Assemblies
Closed and open systems for powder and liquid mixing applications. Options include our exclusive Mixed4Sure™ Bottom Mounted or Top Mounted systems and PVDF-coated stir bars.
Final Filling Assemblies
Featuring Fill4Sure™ filling needles, our filling assemblies are precisely engineered to fit most common filling machines.
Bag Solutions
Discover the versatility of Flex4Sure™ bioprocess bags and assemblies, available in standard 2D, 3D, and custom configurations tailored to your unique needs.
Bottle Assemblies
Featuring PharmaTainer™ bottles and carboys, meticulously crafted from virgin ADCF resins in an ISO Class 5 environment.
Transfer Assemblies
From basic to intricate custom assemblies, we create the perfect fit for transferring crucial bioprocess fluids seamlessly and reliably.
Filter Assemblies
Engineered to deliver consistent and reliable filtration performance across bioprocessing workflows—from development through GMP production—while maintaining material compatibility and process integrity. Configurable with a wide range of filter types, pump-grade tubing, and sterile connection formats.
Centrifuge Tube Sets
Tailored conical-bottle assemblies, including SaniSure’s exclusive Cap2V8® bottle tops, designed to meet your unique specifications.
Flask Assemblies
From basic to intricate custom assemblies, find the perfect fit for transferring crucial bioprocess fluids seamlessly and reliably.
Manifold Assemblies
SaniSure is an industry leader in manufacturing custom non-metallic fabricated fluidic manifolds for biomanufacturing.
Aliquotation
Innovative solutions spanning upstream, downstream, final fill/finish processes, and nearly every critical step in bio-manufacturing workflow.
Sampling Kits
Ready-to-use tubing and connector kits for sampling WFI and other critical media, featuring aSURE™ fused-gasket technology.
Simultaneous Sampling
Draw up to 10 samples with a single connection using our configurable sampling assemblies, made to meet your exact needs wit a multitude of container types, connections and more.
OUR SIX STEPS TO SUCCESS

Problem Identification
We meet to define the problem and target goals.

Collaborative Design
Together we define design goals and objectives.

Engineering Drawing
The design is submitted to our engineering team and we provide you with product drawings.
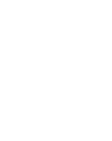
Prototype Creation
Our SMARRT (Speedy Manufacturing Assembly Rapid Response Team) reviews and develops your prototype.

Prototype Testing
You test the prototype and our SMARRT team makes any iterative changes needed.

Final Product Delivery
Your final custom assembly is complete and the product is delivered to you ready to use.
GET MORE INFORMATION
Let’s get started on your fluid management solution. Together we can streamline your operations, reduce risks, and drive growth in your business.
ADDITIONAL SINGLE-USE SOLUTIONS

Single-use, out-of-the-box bottle and carboy small-volume mixing platform.

Bespoke final fill assemblies designed entirely around your drug product.
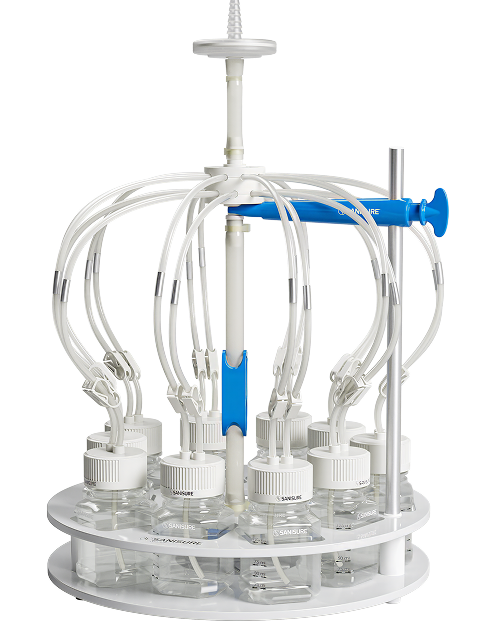
The first simultaneous bottle filling system.
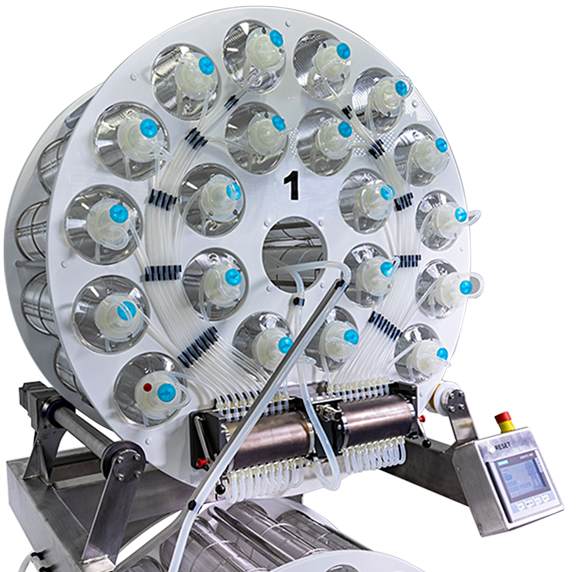
Automated roller bottle processing system for adherent cell culture stages.